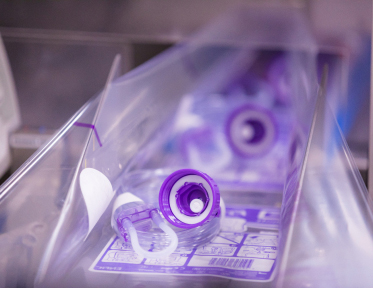
Giving sets, such as Abbott FreeGo and Gravity sets, play a crucial role in healthcare, enabling the safe and accurate delivery of enteral nutrition. The manufacturing process of these giving sets is a highly automated and intricate endeavour, combining advanced technology and stringent quality control measures.
Our products are proudly manufactured at our state-of-the-art facility in Sligo, Ireland, where we have honed our craft in medical device manufacturing for five decades. Throughout this journey, we've evolved and adapted our manufacturing processes to thrive in the precise world of medical device production. Our success is attributed to a strong foundation of knowledge and talent management, placing a paramount focus on engineering precision and cutting-edge automation.
In this article, we will shed light on the meticulous process involved in the manufacturing of Abbott giving sets, emphasising the significance of this process and the materials used to ensure patient safety and product effectiveness.