Protein
Adequate protein intake is essential to help minimise declines in strength and function.34 Protein can also help reduce complications such as infections and can help improve weight.35
THE IMPORTANT ROLE OF MUSCLE MUSCLE LOSS IS COMMON CAUSES OF LOW MUSCLE MASS CONSEQUENCES OF LOW MUSCLE MASS SCREENING, PREVENTION AND TREATMENT IMPORTANCE OF MEDICAL NUTRITIONAL MANAGEMENT RESOURCES
Muscle wasting (with or without fat loss) is a pivotal feature of cancer cachexia, a multifactorial condition that can have a significant impact on patients’ prognosis and quality of life.6
Cancer cachexia often manifests as a significant decline in body weight and changes in body composition. It can lead to severe functional decline and can impact patients' cancer treatment. Cancer cachexia is experienced among patients with many different types of cancer.7
Patients with muscle loss are at increased risk of postoperative complications, treatment toxicity, longer hospitalisation, hospital readmission and poorer prognosis.1,8-10
Sarcopenia is observed in up to:
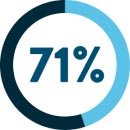
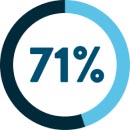
of metastatic colorectal cancer patients11
of head and neck cancer patients*12
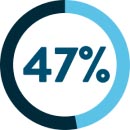
of non-small cell lung cancer patients‡13
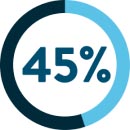
of oesophago-gastric junction cancer patients§14
Following a cancer diagnosis, up to 70% of cancer patients experience loss of muscle mass.1 Evidence shows that cancer patients with pre-treatment weight loss and low muscle mass are at risk of shorter survival and poorer prognosis.15-17
Disease-related causes:2,18-21
Tumours have been shown to increase the release of pro inflammatory cytokines which in turn leads to loss of muscle mass and strength through:
Treatment-related causes:
Chemotherapy can contribute to the onset of cancer cachexia and loss of muscle/muscle strength may persist for after remission.22 Ongoing involuntarily muscle wasting can be caused by the side-effects of treatment, including:3,6,23

Impaired sense of taste and smell leading to loss of appetite

Sickness and diarrhoea
Fatigue and reduced physical activity
Muscle weakness and fatigue caused by cancer treatment such as chemotherapy are some of the most common and distressing symptoms associated with cancer therapy. Muscle loss can impact patients’ quality of life and their ability to physically function, leaving them weak and fatigued.22 The physical deterioration that accompanies cancer cachexia can also leave patients feeling unable or unwilling to eat, which can impact their ability to carry on with everyday life activities.24
Muscle loss and associated weight loss in oncology patients is a predictive factor for:2,3


Muscle loss is also associated with a reduced ability to withstand treatment and can lead to:1,3,4,22


Muscle loss and cancer cachexia can have significant emotional and mental health consequences
Cachexia can also give way to anxiety and stress for the patient. Changes in their physical appearance can challenge their sense of personal identity, independence and health and wellbeing. This is often accompanied by changes in social encounters and this can lead to a heightened sense of loneliness, anxiety and distress.25
Caregivers are also often impacted by their loved one’s anorexia, reduced strength and mobility. Often caregivers do not have an understanding of cancer cachexia, and may encourage their loved ones to eat, which can cause conflict and stress during shared mealtimes, which may have previously been a source of comfort.26
Low muscle mass in cancer patients is reversible, particularly in the early stages of the disease trajectory.3
Evidence suggests that early assessment of muscle function in cancer patients to facilitate appropriate intervention can counteract muscle wastage and prevent cancer cachexia.27
Early identification and management are imperative to help improve prognosis.28 Effective identification of muscle loss and management is particularly important for prehabilitation in order to improve outcomes.5 However, muscle loss can be hard to identify as:29


It can occur prior to weight loss


Up to 60% of cancer patients are overweight
Muscle screening and assessment amongst cancer patients is recommended by the European Society for Clinical Nutrition and Metabolism (ESPEN).28 They often take limited time to apply in clinical practice and are typically non-invasive, inexpensive and convenient for the patient.30,31
Screening can easily identify limited strength and performance, a strong predictor of adverse negative health outcomes and can be easily used to increase physical activity and implement nutrition interventions.30,32
There are several methods to evaluate patients for muscle loss and malnutrition that can be integrated into clinical practice.31
Cancer can be a long and difficult journey and treatments often leave patients feeling tired and weak.22 Early nutritional screening and intervention can lead to improved treatment efficacy, faster recovery and better quality of life.33
Key Nutrients Can Increase Muscle Strength and Improve Quality of Life in Cancer Patients:
Adequate protein intake is essential to help minimise declines in strength and function.34 Protein can also help reduce complications such as infections and can help improve weight.35
HMB is a metabolite of leucine, a branched-chain essential amino acid exclusively obtained from dietary sources.36
Vitamin D acts directly on muscle to promote its function through specific vitamin D receptors found on muscle cells.37 It plays an important role in maintaining musculoskeletal function and can help reduce the risk of falls and improve mobility.38-40


Ensure Plus Advance§ is a 220ml, ready to drink ONS. It contains a unique blend of protein, vitamin D and HMB that improves strength, weight and nutritional status in patients with cancer.°,41 It is the high protein, high energy ONS people prefer to drink vs. market-leading high protein compact**,†,42,43 and achieves 96% compliance.^,44
Validated by more than 18 clinical studies,41,44-62 Ensure Plus Advance is proven to significantly increase strength, mobilisation and wound healing and improve the quality of life in various patient populations.***,††,‡‡,§§,¶,¶¶,~,#,||,41,44,48-50,52,55,57
Abbott’s range of nutritional supplements are designed to meet the different needs of patients with cancer.

Ensure Plus juce
Ensure Plus juce is a 1.5 kcal/ml, ready-to-drink, juice style oral nutritional supplement for people with, or at risk of developing, disease-related malnutrition. Ensure Plus juce is best served chilled and is available in six flavours: apple, fruit punch, lemon and lime, orange, peach and strawberry. It's presented in a 220 ml bottle with a peel-off seal for pouring.

Ensure Compact
Ensure Compact is a 125 ml, ready-to-drink, nutritionally complete§ oral nutritional supplement for people with, or at risk of developing, disease-related malnutrition. Its small volume has been specially developed for people who have difficulty drinking larger volumes or who have a poor appetite. Each easy-to-open Ensure Compact bottle1 provides 300 kcal (2.4 kcal/ml) and 12.8 g of protein.
Ensure Compact is available in 4 delicious flavours; banana, café latte, strawberry and vanilla.
§Nutritionally complete for vitamins and minerals in 625 ml (excluding sodium, potassium, chloride and magnesium). Calculated using the UK Reference Nutrient Intake for men aged 19-50 years.

Vital 1.5kcal
Vital 1.5kcal is suitable for people with disease-related malnutrition and malabsorption or for those who experience symptoms of poor feed tolerance.
On-demand webinar — Prehabilitation: Improving outcomes in cancer care

In this course, Judith Ashcroft, RD describes multimodal prehabilitation and key interventions to improve cancer patient outcomes.
Video: The Prevalence and Impact of Malnutrition, Cachexia and Muscle Loss in Patients with Cancer.

In this video, Michael Bastasch, MD, Martin Chasen, MBChB, FCP, Alessandro Laviano, MD, Carla Prado, PhD, RD, and Şuayib Yalçın, MD, discuss the impact of malnutrition, cachexia and low muscle mass on outcomes in patients with cancer, as well as methods of assessing body composition in this population.
UK-N/A-2300360 | November 2023
Footnotes
HMB - ß-hydroxy-ß-methylbutyrate. *Rounded up from 70.9%. ‡ Rounded up from 46.8%. §Rounded up from 44.9%. § ACBS indication: as a nutritional supplement for frail elderly people (>65 years of age, with a BMI ≤ 23kg/m2), where clinical assessment and nutritional screening show the individual to be at risk of undernutrition. ° Vs baseline: Retrospective database review of 283 patients (63% with cancer) with or at risk of malnutrition who received 2 daily servings of Ensure Plus Advance or standard oral nutritional supplements in combination with dietary counselling and exercise for 3- 6 months. Handgrip strength: 6.2 vs. 4.7 kg; p>0.05. ** Vs. market-leading high protein compact. †243 healthy adults who were asked to drink comparative flavours of Ensure Plus Advance and Fortisip Compact Protein. Approximate values shown. ^ Research with 80 healthy women over 65 years of age supplemented with one serving of Ensure Plus Advance daily for 8 weeks. *** As shown in a randomised control trial to investigate the effects of the intervention ONS on malnourished, cardiopulmonary patients (≥65 years) vs placebo. The intervention ONS decreased mortality at 90 days post-discharge, however the study did not observe a significant effect for the primary composite endpoint of non-elective readmission or death. This post-hoc, sub-group analysis from the NOURISH study cohort comprised 214 COPD patients. ††Strength was measured by handgrip strength in a post hoc analysis of over 600 malnourished people with heart or lung diseases, age 65 or older. Study product was consumed twice a day for 30 days, as compared to standard of care. ‡‡ In 330 older adults with malnutrition and sarcopenia. Muscle quality was calculated as leg strength expressed relative to the muscle mass. §§ As shown in a randomised controlled trial in which normally nourished patients with non-cystic fibrosis bronchectasis received pulmonary rehabilitation plus a specialised ONS or pulmonary rehabilitation only for 12 weeks. In the intervention group, mean and maximum handgrip dynamometry, physical functioning domain of QOL-B-V3.0 and other outcomes were significantly increased from baseline at 12 weeks and 24 weeks and fat free mass at 12 weeks. ¶ An open-label study of elderly (n=35) patients with recent weight loss (>5% during previous 3 months) showed that 12 weeks supplementation of experimental product twice daily increased dietery intake, biochemical variables, and quality of life compared to baseline. ¶¶ In 65 healthy older female patients who regularly attended a fitness programme. ~ As shown in a randomised controlled trial in which normally nourished patients with non-cystic fibrosis bronchectasis received pulmonary rehabilitation plus a specialised ONS or pulmonary rehabilitation only for 12 weeks. In the intervention group, mean and maximum handgrip dynamometry, physical functioning domain of QOL-B-V3.0 and other outcomes were significantly increased from baseline at 12 weeks and 24 weeks and fat free mass at 12 weeks. # In a single arm open-label study of 148 patients aged 80±8.3 years with or at risk of malnutrition who consumed experimental product twice daily for 12 weeks as compared to baseline. || In 92 patients aged 65 and over with hip fractures admitted to a rehabilitation facility, either receiving a standard diet plus 2 bottles of the study product or a standard diet only. Standard diet provided 1500 kcal, 87.4 g protein a day.
References
1. Ryan AM. et al. Proceedings of the Nutrition Society 2016;75:199-211.a. 2. Arends J. et al. Clinical Nutrition 2017;36:1187-11962. 3. Prado CM. et al. Journal of Cachexia, Sarcopenia and Muscle 2020;11:366-380. 4. Cespedes Feliciano E. et al. Proc Nutr Soc 2018; 77 (4):382 387. 5. Macmillan Cancer Support, 2020. Available at: https://www.macmillan.org. uk/healthcare professionals/news and resources/guides/principles and guidance for prehabilitation Accessed: 29th June 2022. 6. Aversa Z. et al. Ther Adv Med Oncol 2017;9(5):369-382. 7. Ni J. & Zhang L. Cancer Management and Research 2020;12:5597-5605. 8. Trejo Avila M. et al. Int J Colorectal Dis 2021;36(6):1077-1096. 9. Lieffers JR. et al. Br J Cancer 2012;107(6):931-936. 10. Real GG. et al. JPEN 2018;42(8):1272-1279. 11. Barret M. et al. Eur J Surg Oncol 2014;66:583–589. 12. Findlay M. et al. J Acad Nutr Diet 2020;120(8):1330-1347. 13. Baracos VE. et al. Am J Clin Nutr 2010;91(suppl)1133S-1137S. 14. Tan BH. et al. Eur J Surg Oncol 2015;41:333-338. 15. Grossberg AJ. et al. JAMA Oncol 2016;2(6):782-789. 16. Chang K V. et al. Liver Cancer 2018;7:90-103. 17. August DA. et al. Journal of Parenteral and Enteral Nutrition 2009;3(5):472-500. 18. Webster JM. et al. Front. Physiol 2020;11:597675. 19. Sharma B. & Dabur R. Curr Med Chem 2020;27(13):2161-2188. 20. Malla J. et al. Cureus 2022;14(7):e26798. 21. Perboni S. & Inui A. Phil Trans R Soc B 2006;361:1281-1289. 22. Pin F. et al. Curr Opin Support Palliat Care 2018;12:420-426. 23. Nolden A. et al. Nutrients 2019; 11 (10): 2285. 24. National Cancer Institute, 2022. Cancer Cachexia: After Years of No Advances, Progress Looks Possible. Available online: https://www.cancer.gov/about-cancer/treatment/research/cachexia#:~:text=Cachexia%2C%20a%20wasting%20syndrome%20that,cancer%2C%20depending%20on%20different%20factors Last accessed November 2023. 25. Hopkinson J. J Cachexia Sarcopenia Muscle 2014;5:89-94. 26. Garcia J. et al. Journal of Cachexia, Sarcopenia and Muscle 2022;13:1418-1425. 27. Dalise S. et al. Eur J Transl Myol 2020;30(2):258-267. 28. Muscaritoli M. et al. Front Oncol 2021;11:682999. 29. Prado CM. et al. Proc Nutr Soc 2016;75:188-198. 30. Beaudart C. et al. Calcif Tissue Int 2019;105,1–14. 31. Cruz-Jentoft A. et al. Age Ageing 2019;48(1):16–31. 32. Murayama I. et al. Aging Clin Exp Res 2020;32, 913–920. 33. Ravasco P. J Clin Med 2019;8(8):1211. 34. Deutz NE. et al. Clin Nutr 2014;33(6):929-936. 35. Cawood AL. et al. Ageing Res Rev 2012;11(2):278-296. 36. Wilson GJ. et al. Nutr Metab 2008;5:1. 37. Wagatsuma A. and Sakuma K. Biomed Res Int 2014;2014:121254. 38. European Food Safety Authority EFSA J 2010;8(2):1468. 39. European Food Safety Authority EFSA J 2011;9(9):2382. 40. Zhu K. et al. J Am Geriatr Soc 2010;58(11):2063-2068. 41. Cornejo-Pareja I. et al. Nutrients 2021;13(12):4355. 42. Data on file. Abbott Laboratories Ltd, 2023 (Ensure Plus Advance acceptability and compliance vs Fortisip Compact Protein). 43. IMS Data, July 2023. 44. Berton L. et al. PloS one 2015;10(11):e0141757. 45. Ashkenazi I. et al. Geriatr Orhop Surg Rehabil 2022;13:21514593221102252. 46. Chavarro-Carvajal DA. et al. Clin Nutr ESPEN 2022;48:291-297. 47. Cramer JT. et al. JAMDA 2016;17(11):1044-1055. 48. De Luis DA. et al. Eur Geriatr Med 2018;9(6):809-817. 49. De Luis DA. et al. Nutr Hosp 2015;32(1):202-207. 50. Deutz NE. et al. Clin Nutr 2016;35(1):18–26. 51. Deutz NE. et al. Clin Nutr 2021;40(3):1388-1395. 52. Ekinci O. et al. Nutr Clin Pract 2016;31(6):829-835. 53. Espina S. et al. Nutrients 2021;13(11):3764. 54. Lopez-Rodriguez-Arias F. et al. Support Care Cancer 2021;29(12):7785-7791. 55. Matheson EM. et al. Clin Nutr 2021;40(3):844-849. 56. Malafarina V. et al. Maturitas 2017;101:42-50. 57. Olveira G. et al. Clin Nutr 2016;35(5):1015-1022. 58. Peng LN. et al. J Nutr Health Aging 2021;25(6):767-773. 59. Previtali P. et al. Ann Surg Oncol 2020;27(6):2025-2032. 60. Ritch CR. et al. J Urol 2019;201(3):470–477. 61. Standley RA. et al. J Gerontol A Biol Sci Med Sci 2020;75(9):1744-1753. 62. Zana S. et al. J Am Med Dir Assoc 2021;22(7):1358-1360.
SESSION TIMEOUT
Please log in to continue
Thanks for registering. To begin, please log in to your ProConnect account. After you log in, you’ll have temporary access to all resources and education while we verify your details.
Added to Bag
ENSURE PLUS
1 x 220 ml
Flavor: Apple
1,5 kcal / ml
Nutrition Information
| Unit | Per {ml-col-1} ml | Per {ml-col-2} ml |
| Unit | Per {ml-col-1} ml | Per {ml-col-2} ml |
| Unit | Per {ml-col-1} ml | Per {ml-col-2} ml |
footnotes
Product details not available.
You have {liteUserDaysLeft} business days of temporary access.
Based on your email address {email} your account has not yet been approved. We’re validating your credentials. You won’t be able to order samples until your account is approved and activated. Please check your email for more details. For help, contact us at abbott.proconnect_access@nhs.net.
UK--2500192 | September 2025
You have {liteUserDaysLeft} business days of temporary access.
Based on your email address {email} your credentials are validated but not yet activated. To gain full access, including the ability to order samples, click the activation link we sent to your inbox. For help, contact us at abbott.proconnect_access@nhs.net.
UK--2500192 | September 2025
Register to continue reading this article
UK-N/A-2400058(v9) | September 2025
You are about to exit for another Abbott country or region specific website.
Please be aware that the website you have requested is intended for the residents of a particular country or region, as noted on that site. As a result, the site may contain information on pharmaceuticals, medical devices and other products or uses of those products that are not approved in other countries or regions.
The website you have requested also may not be optimised for your specific screen size.
Do you wish to continue and exit this website?
UK-N/A-2200465 | July 2022
This website is for UK HCPs and UK Patients only. If you are outside of the UK please visit our global website Abbott.com
If you choose "I am a patient/carer”, you will be entering a website intended for patients and carers which has information on Abbott products. Products on this website are to be used under medical supervision. If you have questions about the product information discussed on this website, you should consult with your healthcare professional.